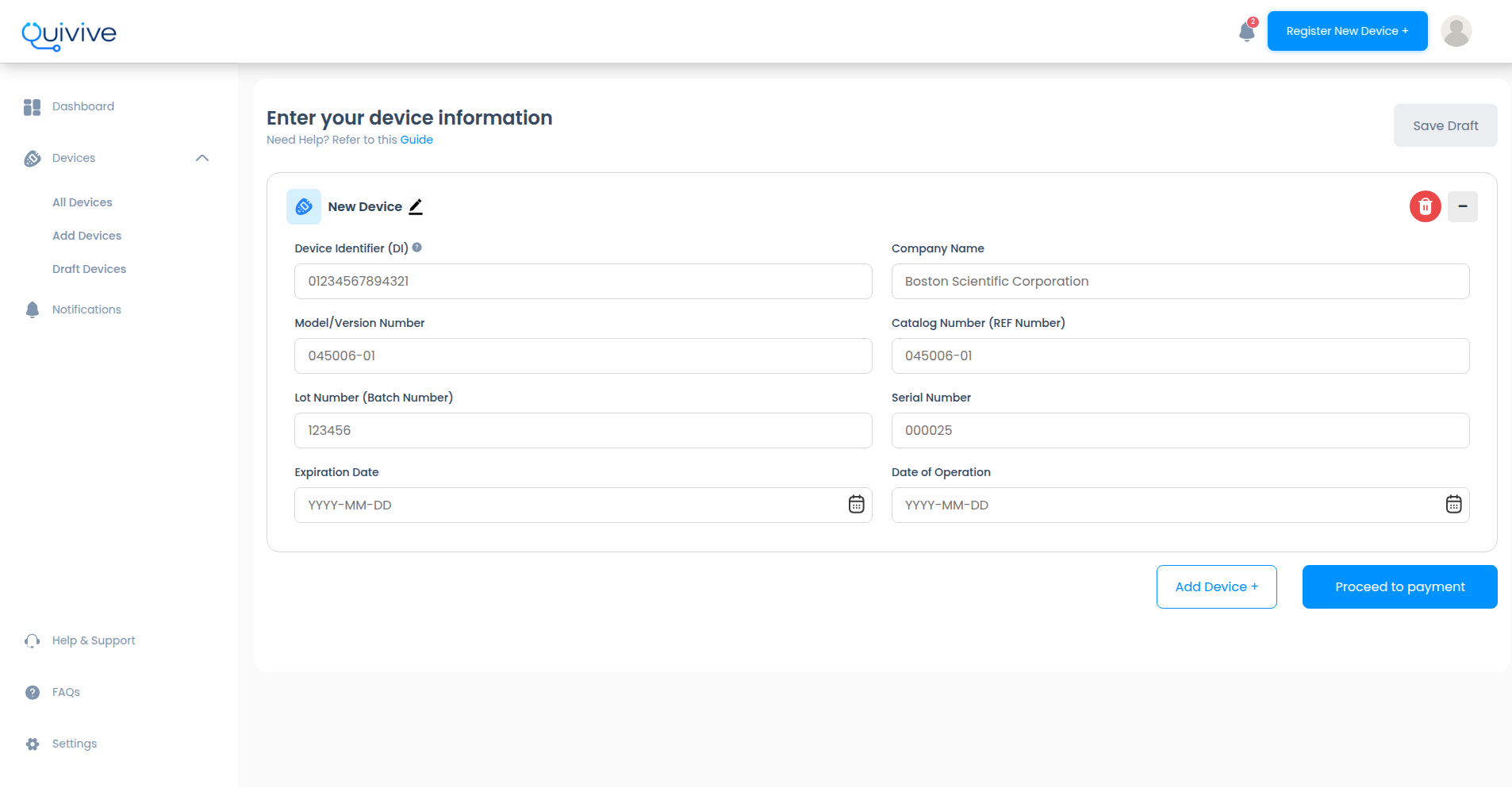
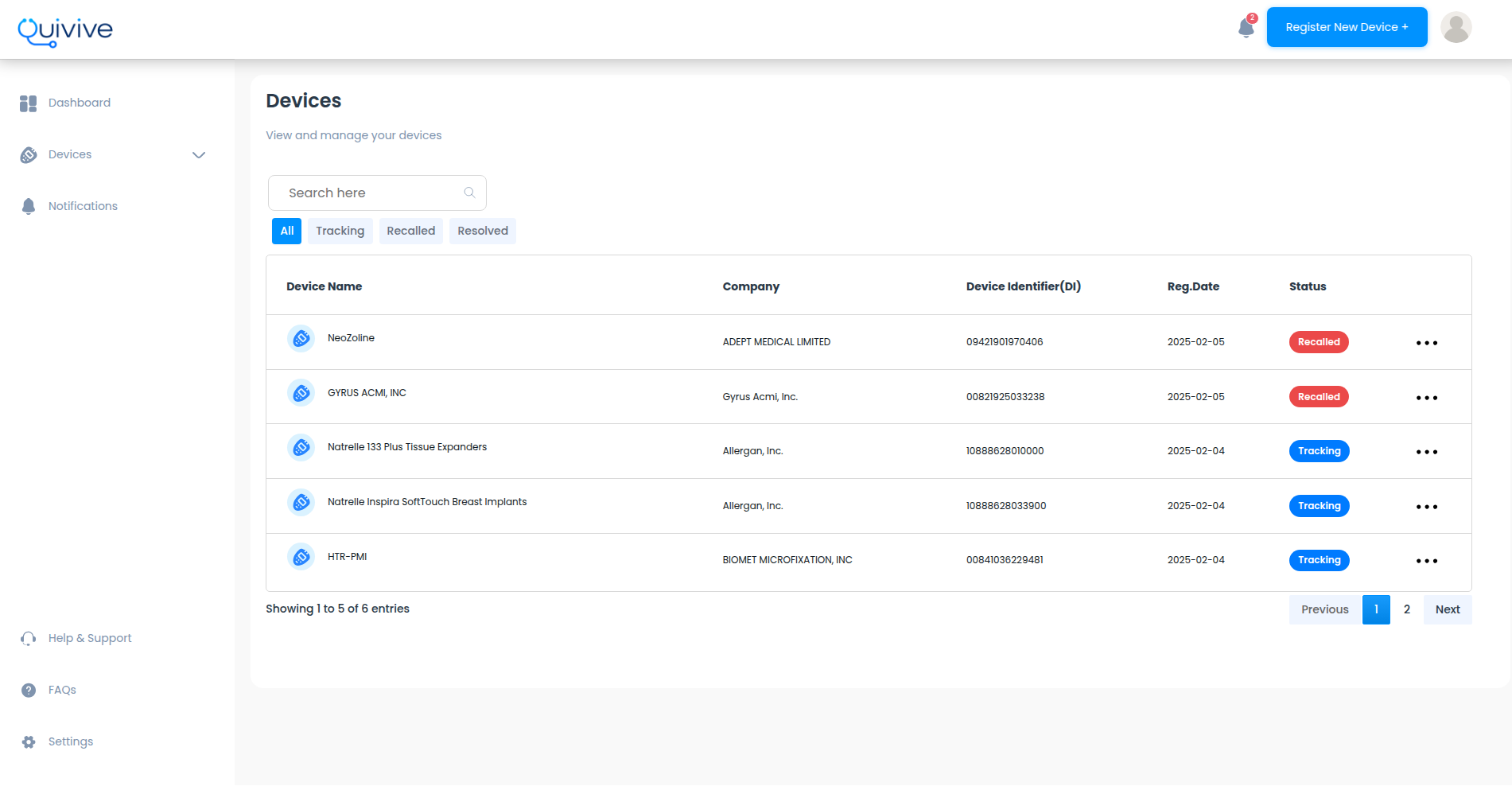
The FDA's approach to recall tracking has made it difficult for healthcare providers to stay informed and keep their patients safe from recalls. Securely register your implant with us and we'll track it for you


The FDA uses antiquated methods which are slow and ineffective in alerting patients and their providers.

Affected patients may be at serious risk depending on the severity of the recall.

It is difficult for patients to keep up with device information.

Standard device identification cards often get lost.
Unique Devices Have Been Recalled, Of Which Millions Of Units Were Produced
Just a few simple steps to get started
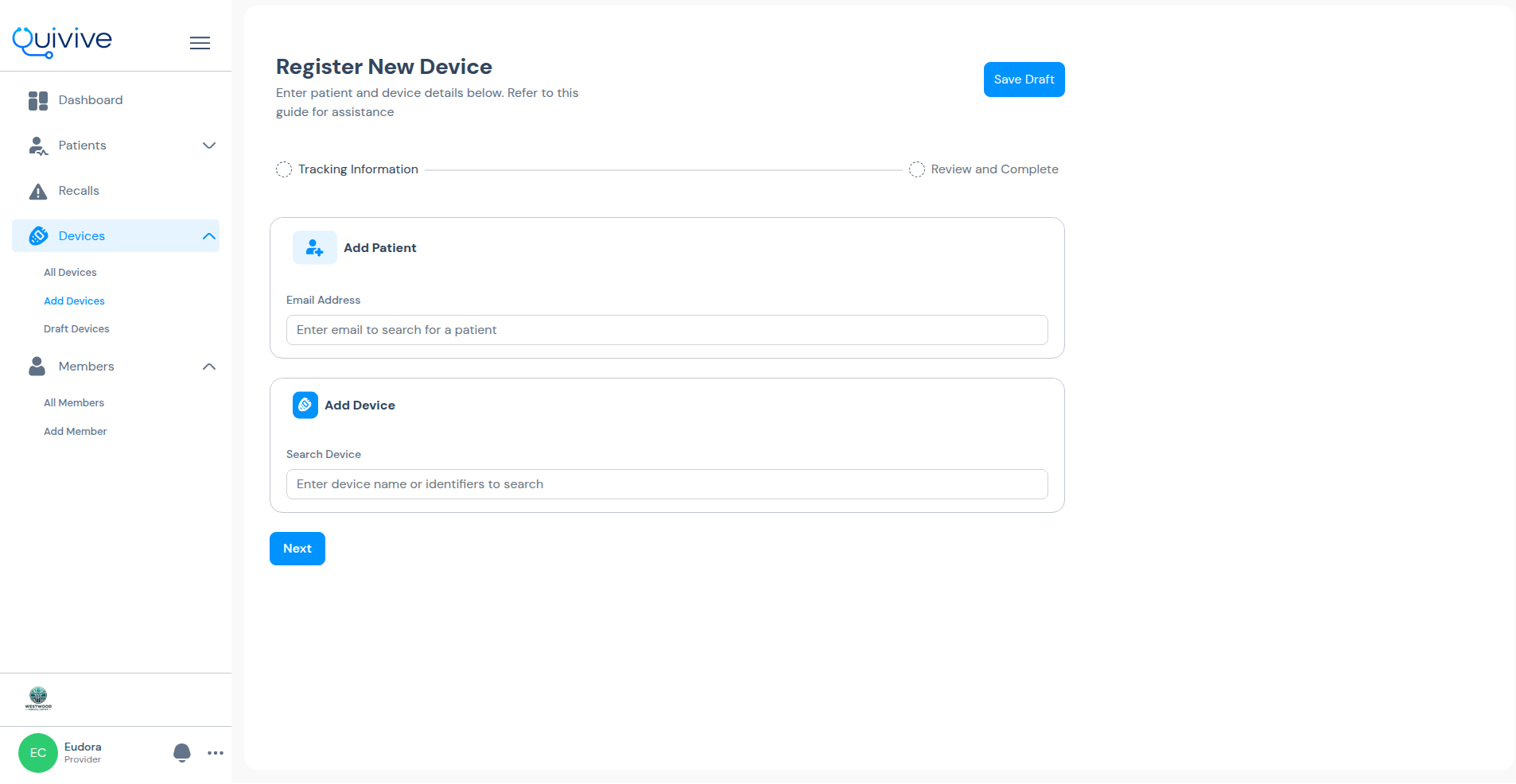
Register your device through our secure web application
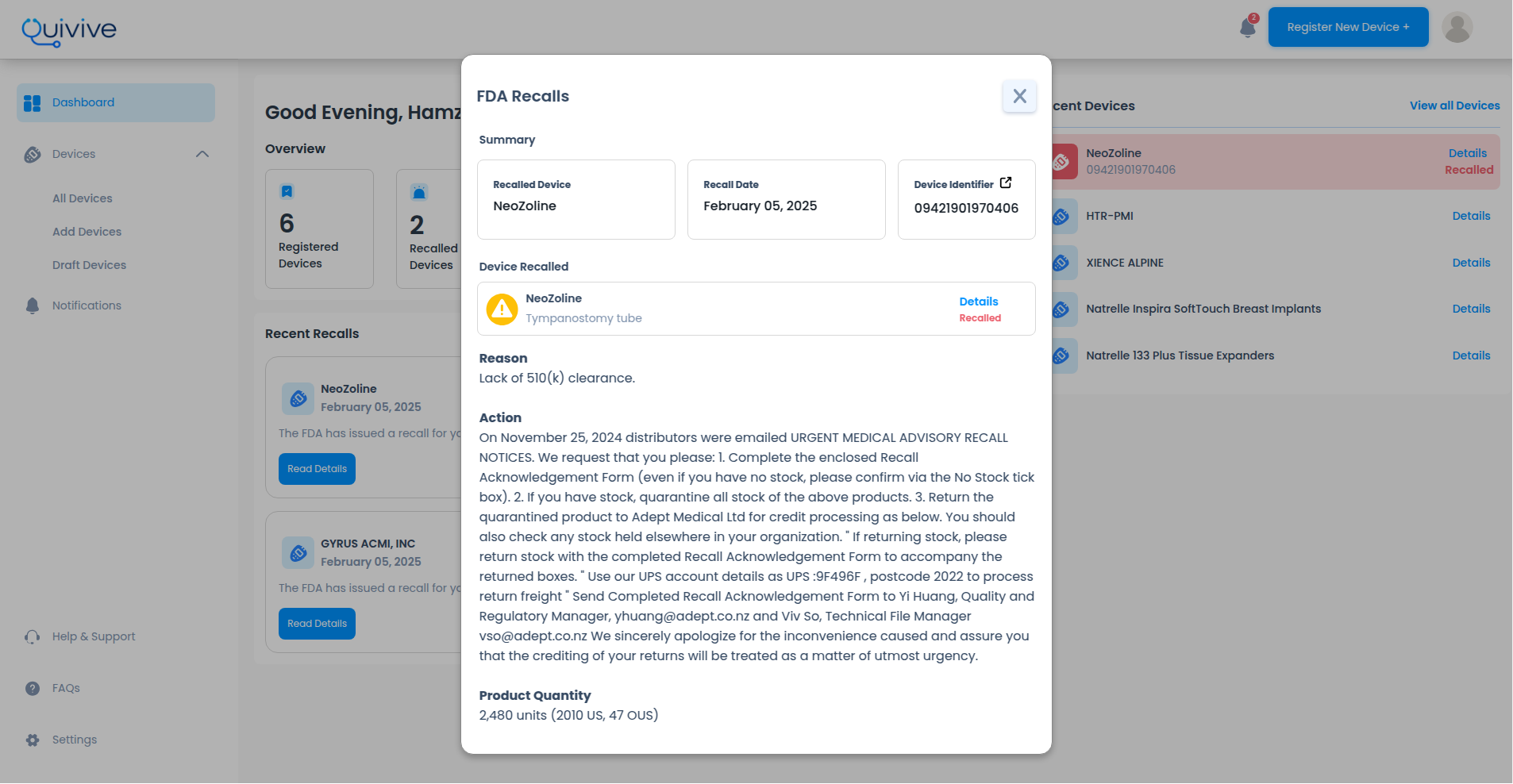
If your device matches a recall record, we'll alert you immediately
We constantly monitor sources for newly recalled devices
What can patients do with Quivive?
Past or recent procedures, we'll track your implants for recalls.
We'll send you an email notifying you as soon as we discover the recall.
Follow up with your provider for the appropriate steps to keep you safe
What can providers do with Quivive?
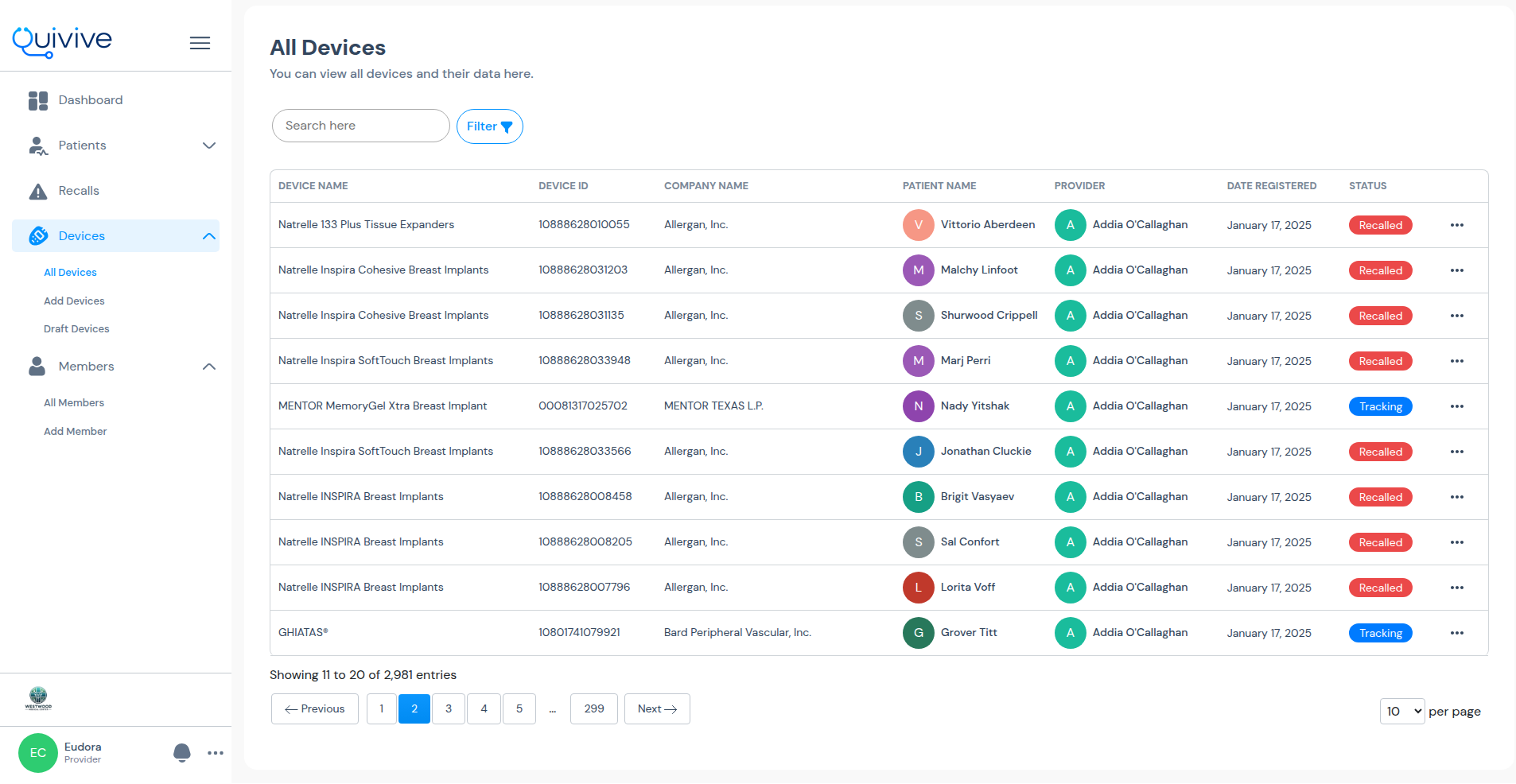
Securely and privately keep records of your patient's implant procedures
Comprehensive dashboard for tracking patient and device recall analytics
In the event of a recall, both patients and providers receive alerts via email and the applications

Keep all medical practitioners together within the organization
HIPAA Compliant through measures such as SSL and Cloud Based solutions
Communicate next steps with the affected patients